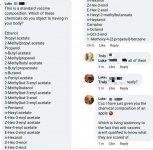
Didn't you tell him that they're injecting people with...a fever....a fever that needs more cowbell??!!
View attachment 85361
As soon as it's your turn, you'll have op-amps and 1n4148 diodes injected straight into your bloodstream, apparently

www.guitarworld.com
While I think that teachers should be on the list of essential workers to be vaccinated, they are still going to run the risk of infection until we figure out how to vaccinate kids. It's looking like this won't happen until next year:
Clinical trials on children 11 and younger "will take much longer, because we have to age de-escalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022," Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay FDA approval, he said.
Vaccines are seen as one of the best ways to stop COVID-19. Learn more about the types of vaccines, including the newly approved Novavax.

www.webmd.com
Please tell your wife to stay safe.
Also
@AnthonyI I'm so glad you're doing better. My heart goes out to you and your family.

www.guitarworld.com
www.webmd.com